A Complete End-to-End Compounding System

Supporting Every Step of Your Workflow
Designed with practicing pharmacists, CurifyLabs brings automation into everyday pharmacy compounding, making personalized medicines practical, scalable, and compliant.
Highlights
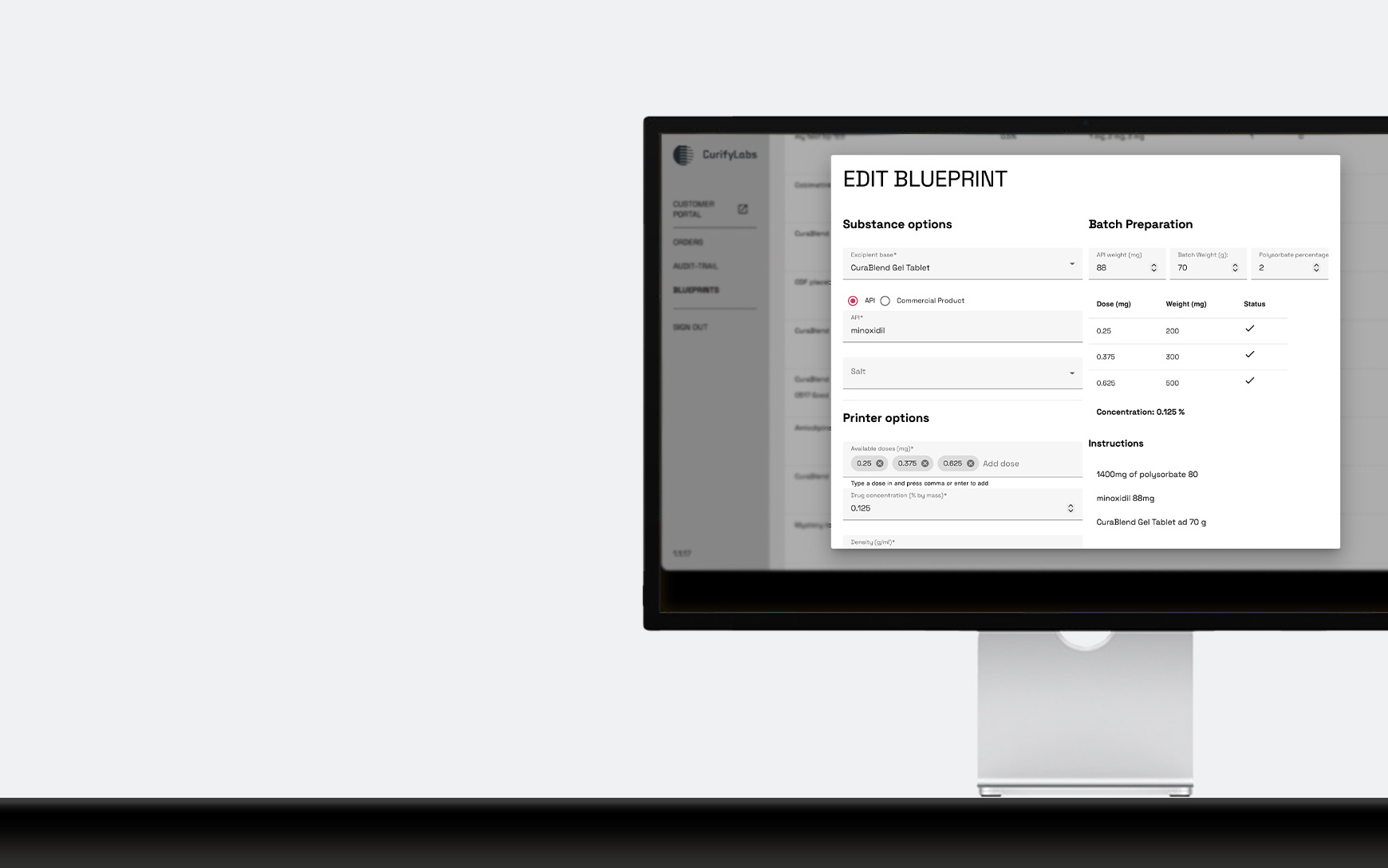
Flexible formulation design for personalized therapies

USP <795>-aligned workflows and documentation to support audit readiness

Multi-dosage-form capability supported by validated excipient bases

End-to-end non-sterile compounding, in one connected system
CurifyLabs Compliance Software & Care Plan

Compliant Digital Workflow with Formulation Library and Ongoing Support
The CurifyLabs Compliance Software & Care Plan combines CurifyLabs software, service support, and formulation library access in one offering. Pharmacies can select validated formulations or design their own, execute compounding workflows with integrated QC steps, and automatically generate compliant digital records, improving consistency, efficiency, and documentation quality.
In addition, the platform provides access to supporting quality documentation for formulations, such as stability studies and dissolution profiles (where applicable), helping pharmacies strengthen internal quality assurance and support audit readiness.
Build the Right Setup Today, Scale as Demand Increases
The CurifyLabs Compounding System is modular, allowing pharmacies to configure the right setup for their operations.
Systems can be expanded over time with additional tools and automation modules, supporting higher throughput, new preparations, and evolving operational needs.
Modeled ROI indicates automation can deliver rapid payback even at lower volumes (e.g., 5–10 prescriptions per day), depending on workflow and utilization.

ECOSYSTEM LEVEL THINKING

ECOSYSTEM
Connected Compounding Ecosystem
CurifyLabs brings pharmaceutical-grade automation into pharmacy compounding, aligned with USP <795> expectations through standardized workflows, integrated quality controls, and compliant digital documentation.
Pharmacists can choose validated formulations from the CurifyLabs Formulation Library or create their own with CurifyLabs Create, supported by integrated QC, digital batch records, and real-time monitoring.
Hardware, software, quality systems, and GMP-manufactured excipients work together as one connected ecosystem, automating the workflow from formulation to end product.
CurifyLabs is an end-to-end solution built for pharmacy practice, combining regulatory compliance with clinical flexibility for personalized medicine.
Validated Formulations with Supporting Quality Documentation
CurifyLabs provides GMP manufactured CuraBlend® excipient bases, including validated formulations, Beyond-Use Dates (BUDs), and quality documentation. This allows compounding pharmacies to adhere to the highest quality standards in compounding in an unmatched way.
Built-In Regulatory Compliance
CurifyLabs system embeds regulatory frameworks directly into the workflow:
- USP <795> -aligned non-sterile compounding procedures
- Validated formulations and digital batch documentation
- Beyond-use date guidance
- Developed under ISO 13485
- Compliant with 21 CFR Part 11 and EU Annex 11 electronic record requirements
- Supports USP <800> for safe handling of hazardous drugs
REGULATORY
CurifyLabs system embeds regulatory frameworks directly into the workflow:
- USP <795> -aligned non-sterile compounding procedures
- Validated formulations and digital batch documentation
- Beyond-use date guidance and ingredient accuracy checks
- Developed under ISO 13485
- Compliant with 21 CFR Part 11 and EU Annex 11 electronic record requirements
- Supports USP <800> for safe handling of hazardous drugs

EXCIPIENT BASES
Validated Excipients with Stability Data
CurifyLabs provides GMP-grade CuraBlend® excipient bases, including validated formulations, Beyond-Use Dates (BUDs), and stability documentation.
Our approach employs non-generic materials and a wider range of formulation examples to ensure reproducibility.

LABELING
Packaging That Protects Quality and Supports Compliance
Pharmacies gain automated unit-level labeling linked to batch records, heat-sealed blister packaging that reduces moisture and oxygen exposure, and configurable GMP-compliant packaging options.
This integrated workflow improves traceability, reduces variability, and delivers more consistent product quality with fewer defects and patient complaints.

Product Offering
Our Hardware

Our Software
Our Excipient Bases
End-To-End Compounding

By combining hardware, software, excipients, compliance, and QC into a single, coherent system, pharmacies can achieve:
- Higher safety and compliance
- Faster time-to-dispense
- Reduced manual workload
- Less training and fewer errors
- Scalable, reproducible personalized medicine
MANUFACTURING
End-To-End Manufacturing Support
By combining hardware, software, excipients, compliance, and QC into a single, coherent system, pharmacies can achieve:
- Higher safety and compliance
- Faster time-to-dispense
- Reduced manual workload
- Less training and fewer errors
- Scalable, reproducible personalized medicine
